Abstract
Introduction: Most venous thromboembolism (VTE) in children are provoked and have an overall low risk for recurrence, especially if the original provoking factor is mitigated. Nonetheless, certain factors heighten VTE recurrence risk, including hospitalization. In fact, the rate of VTE in hospitalized children has increased more than 200% since 20011. This staggering discovery emphasizes the dire need for more effective tactics aimed at VTE prevention. Historically, pediatric providers have been hesitant to initiate pharmacologic (i.e. anticoagulant) measures in children for a number of reasons. In our recent survey of practice patterns of secondary prevention against recurrent VTE in pediatrics, we found low use (<25%) of pharmacologic prophylaxis in high-risk children2. Additionally, most respondents did not feel comfortable initiating secondary pharmacologic prophylaxis in younger children2.
In the wake of the COVID-19 pandemic, numerous trials have been conducted to evaluate anticoagulation for VTE prevention in affected individuals. The recently published COVID-19 Anticoagulation in Children- Thromboprophylaxis trial showed safety of low-dose enoxaparin in children hospitalized with COVID-19 or multisystem inflammatory syndrome in children (MIS-C)3. Furthermore, trials of direct oral anticoagulants have also shown safety and efficacy in secondary VTE prevention in children4. Importantly, these studies have consistently demonstrated low rates of bleeding.
Methods: The CHAT Registry is an IRB-approved registry that captures clinical data on hospital-acquired VTE in children <21 years of age and non-VTE controls. Subjects were enrolled from 2012-2021 across 8 participating pediatric hospitals. Using this REDCap database, we performed a retrospective study of enrolled subjects to explore trends for secondary anticoagulation prophylaxis use in hospitalized children. Subjects (cases and controls) were included if they had a history of VTE. Descriptive statistics were used to analyze demographics/characteristics and VTE prophylaxis measures.
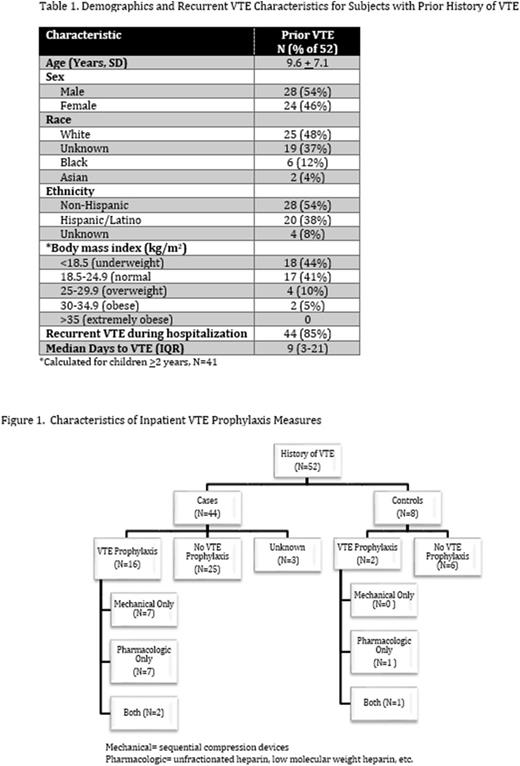
Results: Fifty-two subjects (%) out of 2219 total CHAT subjects (1273 cases, 946 controls) reviewed had a prior history of VTE. Characteristics of these subjects are presented in Table 1. The mean age was 9.6 years with a slight male predominance (54%). Regarding VTE prophylaxis (Figure 1), 18 (35%) received VTE prophylaxis (mechanical and/or pharmacologic) during hospitalization. Of these, 11 subjects (61%) were prescribed pharmacologic prophylaxis either alone (8) or in combination with mechanical prophylaxis (3). The remaining seven (39%) received only mechanical prophylaxis. Four subjects were already on anticoagulation at the time of admission.
Forty-four (85%) subjects experienced a hospital-acquired VTE recurrence. Only 16 (36%) of these had received some method of VTE prophylaxis. Of those who did not receive pharmacologic prophylaxis, only 3 had a documented contraindication. The most commonly documented contraindications to pharmacologic prophylaxis pertained to risk of bleeding, and included uncorrected coagulopathy, ongoing/uncontrolled hemorrhage and thrombocytopenia. The median time between admission and VTE event was 9 days.
Conclusion: There is a high rate of recurrent VTE in hospitalized children with a history of prior VTE. These results underscore the significance of implementing consistent pharmacologic prophylaxis in high-risk children where appropriate. As the landscape is actively changing in terms of available anticoagulant formulations available for children, providers may feel more confident in initiating secondary pharmacologic prophylaxis particularly as new data confirm the safety. Nevertheless, ongoing definitive studies are required to better understand which particular factors yield the highest rates of VTE and hence warrant pharmacologic prophylaxis above standard mechanical measures. We want to acknowledge the CHAT Consortium sites who contributed to the CHAT Registry.
Disclosures
Jaffray:Bayer: Consultancy. Lebensburger:Novartis: Consultancy; Forma: Consultancy; Agios: Consultancy; Bioproduct Laboratory: Consultancy. Goldenberg:Boehringer-Ingelheim: Consultancy; Daiichi: Consultancy; BMS/Pfizer: Consultancy; Bayer: Membership on an entity's Board of Directors or advisory committees; NIH NHLBI: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal